To safeguard consumers from counterfeit, contaminated, or adulterated prescription drugs unfit for U.S. distribution, the U.S. Congress enacted the Drug Supply Chain Security Act (DSCSA) in 2013. DSCSA compliance ensures that all players in the pharmaceutical supply chain meet stringent DSCSA requirements for pharmacies and other entities, enhancing overall safety and security.
The DSCSA requires that every prescription drug package and homogeneous case have its own unique identifier in machine- and human-readable forms. These DSCSA compliance pharma requirements include:
Additionally, each identifier must be stored in a 2D Datamatrix barcode, supporting electronic interoperability to trace prescription drug products across the supply chain.
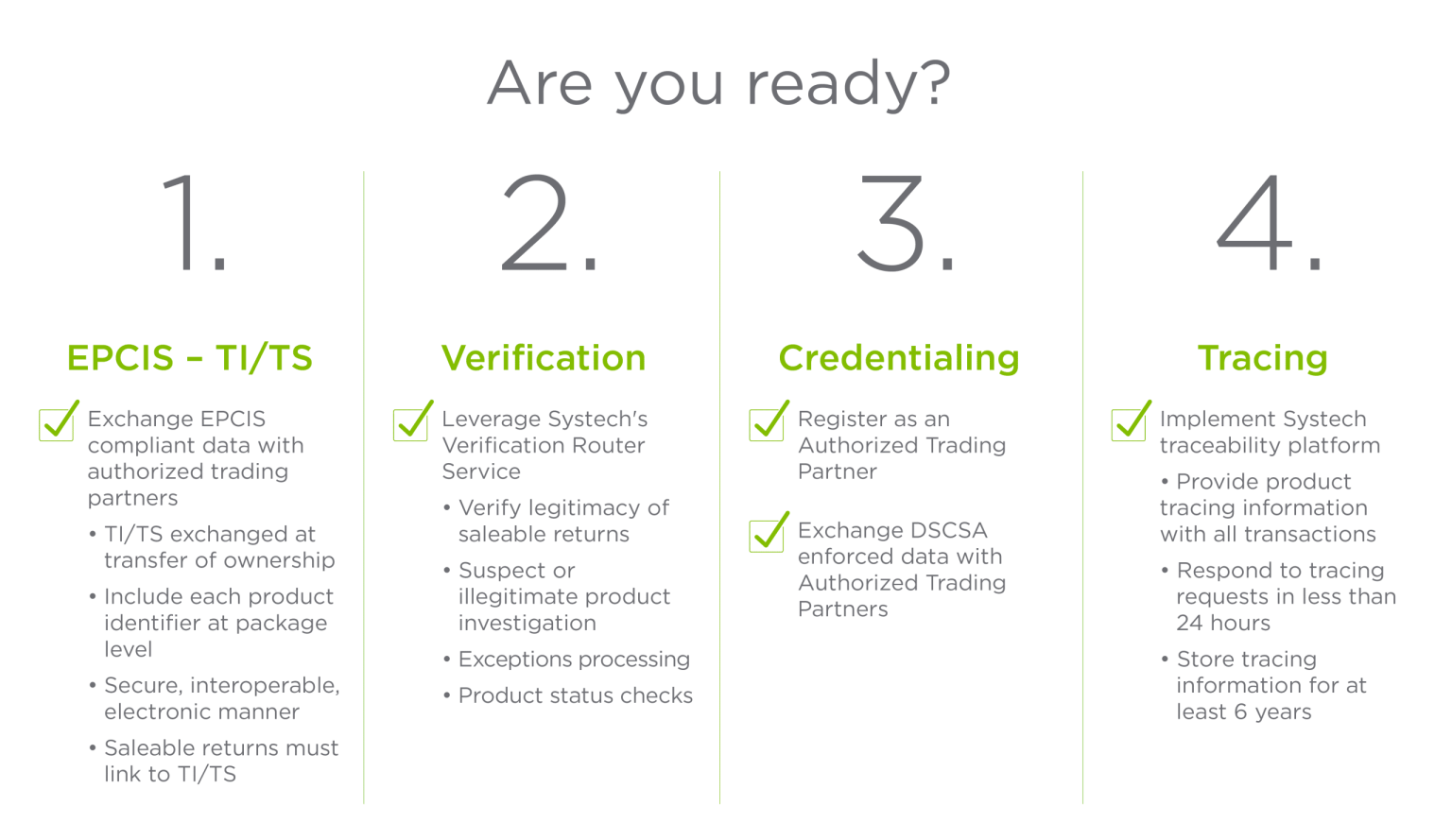
Though the official DSCSA deadline for interoperability compliance—November 27, 2023—has passed, the FDA has recently introduced recent exemptions in the form of a phased implementation plan for DSCSA. During this period, it is crucial that pharmacies and other supply chain entities meet DSCSA requirements for pharmaceuticals and ensure compliance before the extended deadline. We can help.. As the global leader in serialization, Systech offers the speed, flexibility and proven expertise needed to get you to compliance. Together, we’ll implement solutions.
Implement exceptions management solution to ensure the physical matches the digital.
As a leader in serialization, Systech provides a complete traceability solution for everyone in the supply chain ecosystem—manufacturers, contract packagers, distributors, 3PLs, dispensaries and regulatory bodies. Our proven implementation process is configurable, scalable and built to meet the integration and compliance requirements of today and tomorrow.

Systech is proud to be recognized as a VRS provider committed to the Open Credentialing Initiative
“OCI is an open initiative enabling trusted digital interactions between Authorized Trading Partners (ATPs) in the U.S. pharmaceutical supply chain to meet the requirements of the U.S. Drug Supply Chain Security Act (DSCSA).”
Learn all about DSCSA stabilization period and what it means for compliance. Get in touch with a compliance expert today!
Takeaways from the recent 2024 FDLI Annual Conference with focus on readiness for DSCSA…
Insights into the current state of affairs and opportunities offered by DSCSA stabilization period….
As part of the FDA’s Drug Quality and Security Act, one key rationale for the Drug Supply Security Act (DSCSA)…