The EU’s Falsified Medicines Directive (FMD) has those involved in the commercial pharmaceutical supply chain thinking about serialization and track and trace systems. For clinical trial supply chains, the focus is more on individual product labeling. That encompasses both printing labels and verifying the label content, both of which play significant roles in meeting regulatory requirements.
In Part 1 of this two-part blog series, we discuss the challenges involved in labeling the medicinal products used in clinical trials. In Part 2, we will discuss solutions for overcoming those challenges, including the use of Systech’s T11 Manual Mark & Verify
The EU regulations for labeling investigational medicinal products (IMPs) and auxiliary medicinal products (AMPs) used in clinical trials in the pharmaceutical industry are stringent — and for good reason. The labels provide clinical trial administrators and subjects with critical information such as dosage, administration instructions, storage direction, and expiry. Any label errors can jeopardize participant safety and the integrity of the clinical trial data.
Complying with labelling regulations is not always easy. The requirements can be extremely complex, especially when clinical trials are being conducted in multiple countries. Most countries require AMP and IMP labels and other information to be provided in their preferred language or languages. Each country, as well as the specific site locations, may also have their own labelling requirements.
Further complicating clinical trial labelling is the fact that any updates to the medicinal product or its handling during or between clinical trial phases necessitate label changes. For example, new information on IMP stability often emerges during clinical trials. When that happens, the IMPs must be relabeled with updated expiry dates and other content.
Any change opens up the opportunity for errors, reinforcing the importance of label verification in the printing process. While the benefits of electronic labels (eLabels) have the potential to mitigate errors, the EU still requires that IMP labels be printed.
In addition, print runs of labels used in clinical trials are typically small compared to those for standard pharmaceutical labels. That makes investment in large integrated printing and verification systems impractical, not to mention that most organizations running clinical trials lack the space for large-scale equipment. Outsourcing is an option, but the need to accommodate frequent changes in label content make that an inefficient choice and increases the risk of delays.
As if organizations did not have enough to deal with in terms of labels for clinical trials, they now must also contend with the European Union Clinical Trial Regulation 536/2014 (EU CTR). While it is intended to harmonise the rules for conducting clinical trials throughout the EU, it also adds confusion.
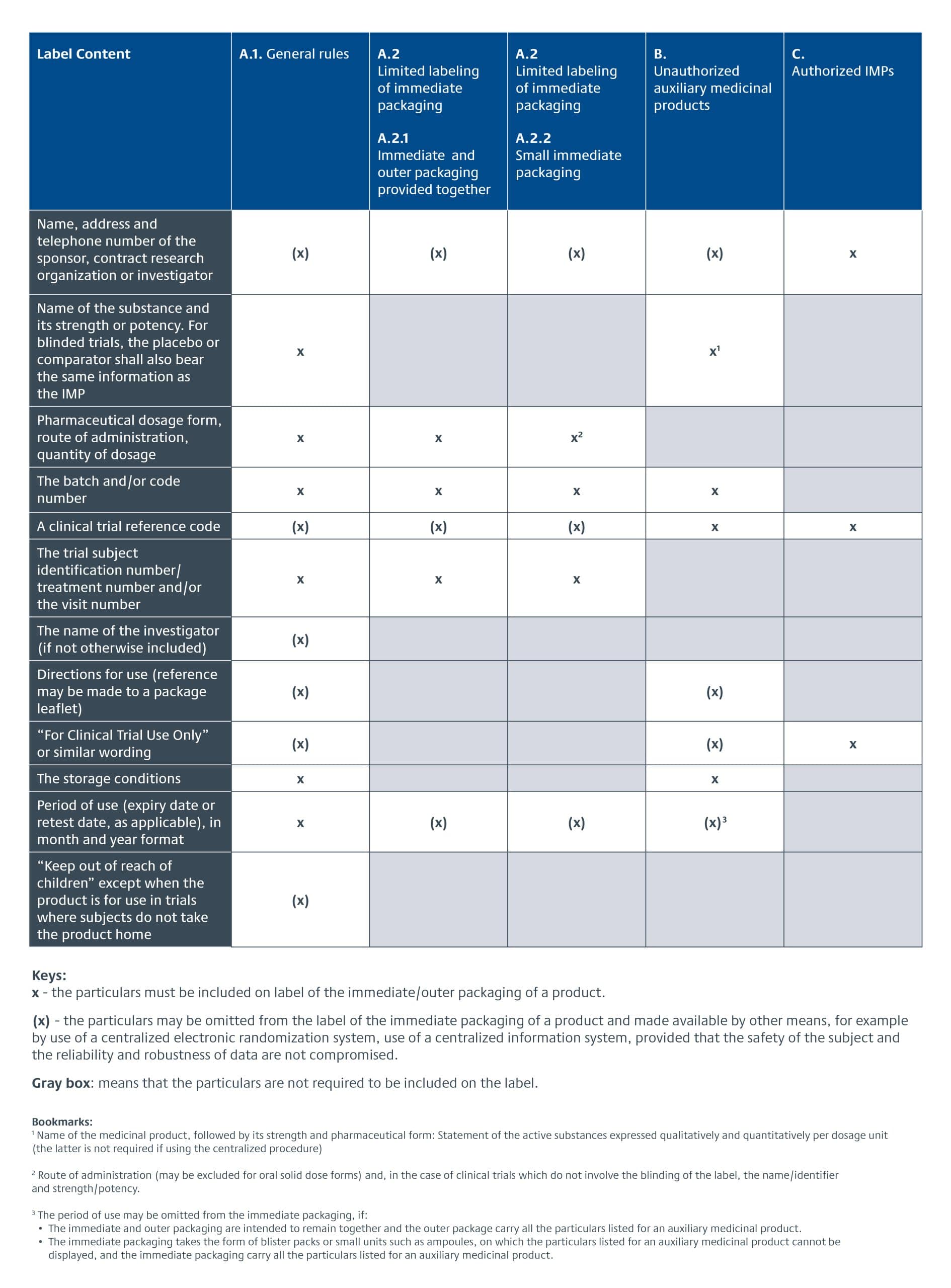
The EU CTR went into effect 31 January 2022, replacing the European Union Clinical Trial Directive (EU CTD) 2001/20/EC. EU product labeling requirements were changed from being part of the GMP requirements described in Annex 13 for Investigational Medicinal Products (sections 26-33) to being part of the legislative text of the CTR, Articles 66-70. The specific requirements for investigational product labeling for EU clinical trials are detailed in Annex VI of the EU CTR. There is a three-year transition period for adopting it. The following provides an overview of the IMP labelling requirements under EU CTR.
In addition, the new Clinical Trials Information System (CTIS), went live on 31 January 2023. It is now the single entry point for submission of data and information relating to clinical trials required by EU CTR. Sponsors of clinical trials had one year to transition to the new system.
The greatest confusion lies in terms of whether EU CTR or EU CTD applies to IMP labelling requirements. Clinical trials submitted under EU CTR must comply with the new label requirements.
As of 31, January 2024 — the end of the transition period to CTIS — all clinical trial submissions must be submitted through the CTIS and are subject to the EU CTR regulations. Existing clinical trials authorized under EU CTD, which could include any submitted prior to 31, January 2024, can keep their original labeling.
Yet another factor to consider regarding labelling requirements for clinical trials: the qualified person (QP). Under European Union (EU) law, the QP certifies that each batch of a medicinal product meets all required provisions when released from a manufacturing facility within the EU, or imported into the EU. While the EU CTR has been adopted, how it is interpreted can vary from one QP to another. It is imperative for the medicinal product sponsor and QU to agree to the labelling strategy and content to prevent compliance issues and timeline delays.
There is also an amendment to Annex VI that was adopted in September 2022 and made effective upon publication in the Official Journal of the European Union on 15 November 2022. It eliminates the requirement to include expiry on the immediate packaging of some IMPs used in clinical trials.
Despite the challenges, the ability to produce error-free labels as needed and when needed is critical. Keep in mind that:
In part 2 of this blog series, we will discuss some of the options for producing clinical trial labels and the features to look for that can meet the criteria noted above.