Achieving traceability is essential for manufacturers to gain full supply chain visibility and meet compliance, resiliency and sustainability goals. It begins by capturing accurate product data at the packaging line and extends through the entire supply chain, creating a digital representation of real-world product flows.
Systech offers a cloud-based solution for consolidating, sharing and reporting product data from various sources across diverse supply chain ecosystems, ensuring complete traceability. Our solution provides:
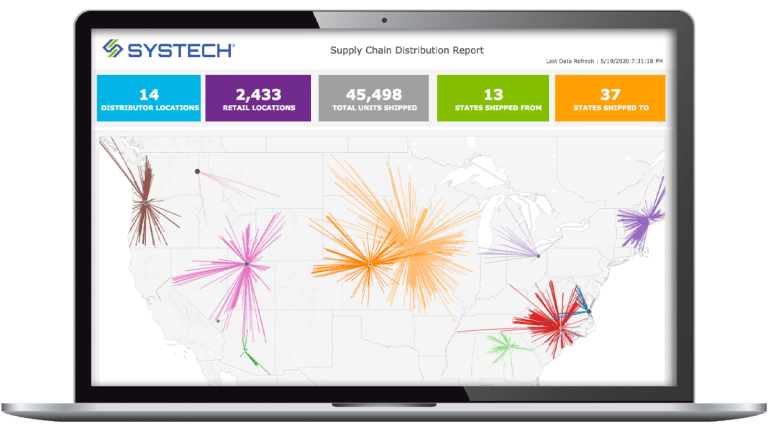
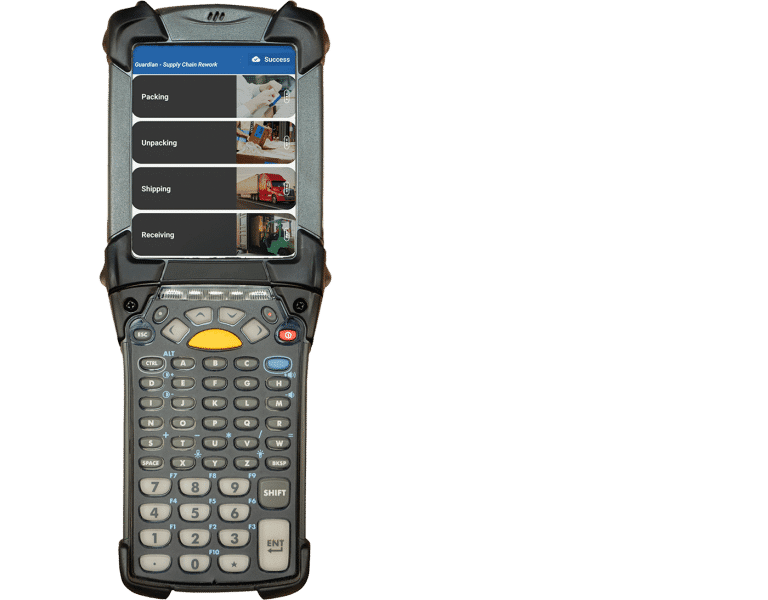

With Systech, you can capture data on key supply chain metrics to measure and enhance performance from manufacturing to the marketplace. The platform integrates logistics, rework, and exception handling without the need for third-party software, ensuring end-to-end traceability.
Traceability provides both real-time visibility and historical insight into your supply chain to help avoid past mistakes, plan for future needs and become more responsive to industry, government and consumer demands.
By enabling you to track and share data, and get constant feedback, traceability empowers you to improve processes and collaboration in support of business objectives such as:
With traceability, your business gains both real-time visibility and historical insight to improve process optimization, risk mitigation, regulatory compliance and consumer safety. Systech empowers everyone in the supply chain ecosystem—manufacturers, distributors, 3PLs and regulatory bodies—with the tools to streamline operations and meet industry demands.
Owning your traceability data is the foundation for future-proof supply chains. The time to start is now…
Protecting brand integrity is a multifaceted discipline which can be complicated by counterfeiting and…
Learn how pharma companies can optimize packaging operations and leverage their supply chain data.