Implementing a new serialization process is no small task. At Systech, we know what it takes to help you successfully deploy the best L1-L3 solutions to meet ever-changing market requirements, compliance regulations and a broad range of packaging environment challenges.
You are responsible for deploying a serialization solution that will meet short- and long-term business needs. Our platform is line equipment agnostic and readily configurable, giving you the flexibility needed to adapt to new business and regulatory requirements quickly and cost-effectively while maximizing uptime.
How? We leveraged four decades of experience with hundreds of customers and thousands of line implementations to create a repeatable methodology for a wide variety of environments. Our proven approach is efficient, scalable and economical—designed to minimize line downtime and ensure operational stability.
Check out this guide to learn more about our approach to configurable software, and how we help you comply with Good Manufacturing Practice (GMP).


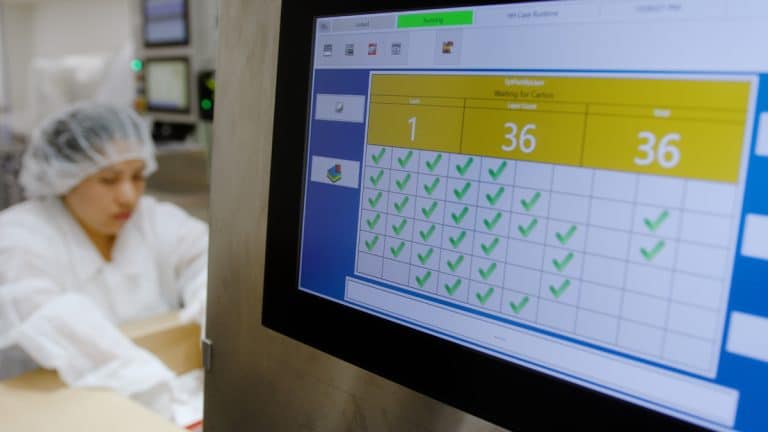

When implementing serialization, you need to be mindful of the complete costs. Beyond the initial software, hardware, systems integrations and validation, it will require maintenance and changes as your business and industry regulations evolve. These costs are often underestimated.
Systech solutions are highly scalable—and operating within a majority of the world’s leading pharmaceutical manufacturers, CMO, 3PLs and CPOs. We are the only provider that can offer you:
You will benefit from comprehensive support and guidance throughout the implementation process and beyond. We partner with you to ensure your serialization investment is protected and maximized.
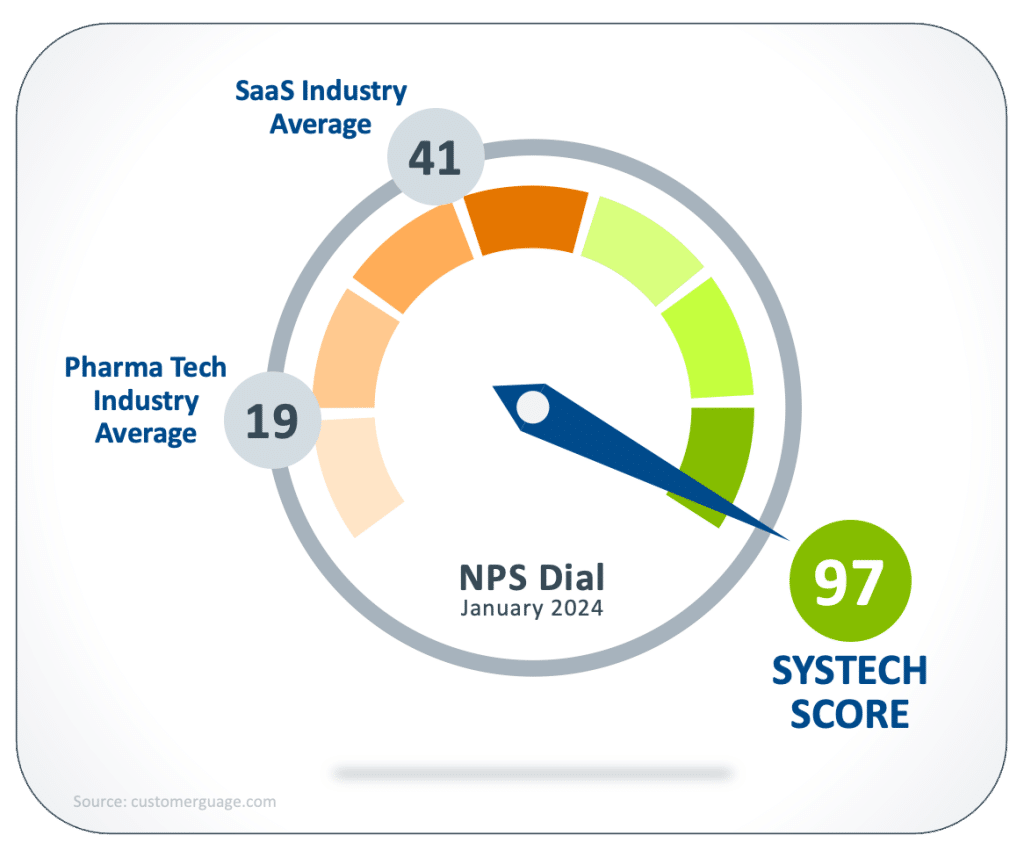
If your current serialization solution is faltering, we know that changing providers is easier than you might think. Systech replaced an incumbent vendor at three Boehringer Ingelheim sites, in two countries, validated and in full production within six months.
Our platform is:
It’s time to partner with a market leader that offers faster setup, better control over the serialization process, outstanding support and the ability to drive down TCO via greater efficiency.
International pharmaceutical manufacturer Takeda faced the task of implementing serialization on over 60 globally distributed packaging lines.
Systech delivers supply chain solutions that our customers trust to bring billions of critical products to market safely.
Find best practices to select and design an effective aggregation strategy that works seamlessly to keep your products moving.