Sérialisation. Agrégation. Emballage. Suivi et traçabilité. Authentification des produits.
Les CMO, CDMO et CPO du monde entier se tournent continuellement vers Systech pour obtenir une agilité inégalée, une conformité aux réglementations mondiales et une évolutivité rapide dans n'importe quel environnement d'emballage.

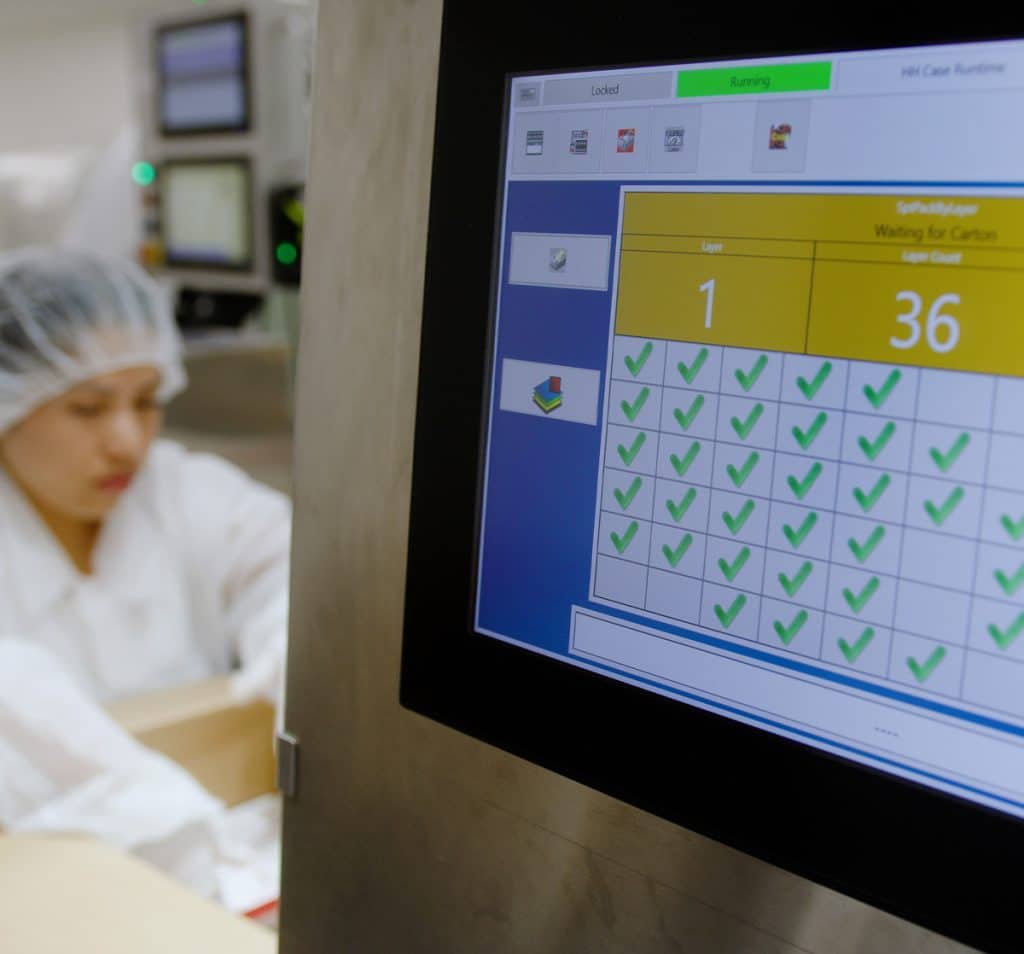


Les clients potentiels nous demandent : "Qui est votre fournisseur de sérialisation ?". Une fois que nous avons répondu "C'est Systech", plus personne n'a d'inquiétude. Je ne peux pas en dire autant si nous travaillions avec un autre fournisseur.
- PDG, Nutra-med Packaging
"Lorsque vous avez un seul fournisseur ou prestataire de services à toutes les étapes - avec la sérialisation, l'agrégation, le suivi et la traçabilité - la vie est plus simple.
- COO, Leading Pharma
"Je recommande vivement Systech pour son expertise, sa fiabilité et son souci d'excellence, en particulier dans le domaine de l'emballage pour les services pharmaceutiques et les CDMO.
- SVP, Sharp Packaging